Nazan Saraç Çoban, Nil Toplan
Sakarya Üniversitesi, Metalurji ve Malzeme Mühendisliği
*Perlit ve Kuvars Kullanılarak Atmosfer Basıncında Kurutma ile Silika Aerojel Sentezi
 ABSTRACT
ABSTRACT
Silica aerogel is synthesized by drying atmospheric pressure using natural silica raw materials source such as perlite and quartz. Due to the porous structure and the unique microstructure of nanometer sized particles, there is a wide number of application areas of silica aerogels. The resulting aerogels are sol-gel materials which dried to prevent pore collapse. The silica source powder was dissolved by boiling in the sodium hydroxide solution for a specified time. After filtration, the ph neutralized solution was allowed to age at room temperature. Surface modification and drying in gel aging process are the most critical steps in sol-gel production. By surface modification with TEOS (tetraethylorthosilicate) the bonds in the structure are strengthened and the material strength is increased. Silica gels were treated with solutions prepared using ethanol and TEOS chemicals to support the aging phase. Solvent exchange with n-heptane was carried out before drying in atmospheric pressure and finally silica aerogel powders were dried for 1 day at 50, 75 and 125oC at atmospheric pressure. SEM, FESEM, XRD, FTIR, DSC/TG and BET analyzes were performed on the obtained silica aerogel powders and the results were discussed. As a result of the experimental studies and analyzes, it has been seen that silica aerogels with superior properties are synthesized by the atmospheric pressure drying technique.
ÖZET
Silika aerojel doğal silika kaynağı olan perlit ve kuvars gibi hammaddeler kullanılarak atmosfer basıncında kurutma ile sentezlenmiştir. Gözenekli yapısı ve nanometre boyutundaki parçacıklardan oluşan özgün mikroyapısı nedeniyle silika aerojellerin çarpıcı sayıda uygulama alanı bulunmaktadır. Elde edilen aerojeller gözenek çökmesini önleyecek şekilde kurutulmuş sol-jel malzemelerdir. Silika kaynağı olan tozlar, sodyum hidroksit çözeltisinde belirlenen sürede kaynatılarak çözünmesi sağlanmış ve filtre edildikten sonra ph’ı nötralize edilen çözelti oda sıcaklığında yaşlanmaya bırakılmıştır. Jelin yaşlandırılması evresindeki yüzey modifikasyonu ve kurutma sol-jel üretimindeki en kritik aşamalardır. Silika aerojel sentezinde kullanılan TEOS (tetraethylorthosilicate) ile yüzey modifikasyonu sağlanarak yapıdaki bağlar kuvvetlendirilmiş ve malzeme dayanımı arttırılmıştır. Jeller, etanol ve TEOS kimyasalları kullanılarak hazırlanan çözeltiler ile muamele edilerek yaşlandırma evresi desteklenmiştir. Atmosfer basıncında kurutmadan önce, n-heptan ile çözücü değişimi yapılmıştır ve son olarak atmosfer basınçta 50, 75 ve 125oC’de 1 gün boyunca silika aerojel tozlarının kuruması sağlanmıştır. Elde edilen silika aerojel tozlarına SEM, FESEM, XRD, FTIR ve DSC/TG cihazları ile analizler yapılarak sonuçlar tartışılmıştır. Yapılan deneysel çalışmalar ve analizler sonucunda atmosfer basıncında kurutma tekniği ile üstün özelliklere sahip silika aerojellerin sentezlendiği görülmüştür.
 1. Introduction
1. Introduction
Aerogel is an open-pored material with the lowest density among solid porous materials. Due to its superior properties such as low density, low thermal conductivity, high surface area and high porosity, it is used in many sectors [1]. The most commonly aerogels are used thermal insulation raw materials, catalyst and catalyst fillers and adsorbent materials [2]. Silica aerogels have the greatest potential for thermal insulation applications because of their ability to achieve temperature-sensitive and energy-efficient products, and the thermal conductivity of these synthesized products is extremely low [3].
Silica aerogels are generally synthesized using expensive and environmentally hazardous starting materials. Various studies are being made to manufacture using environmentally friendly natural aerogel sources to remove this disadvantage. Another important and critical factor synthesis is the drying process in aerogel. Supercritical drying leads to a very expensive and large amount of energy use while increasing the cost. Two methods can be proposed to reduce the production price. The first is to use cheaper and natural silica aerogel sources, and the second is to perform the drying process under atmospheric pressure conditions [4,5].
The sol-gel method is widely used in the synthesis of silica aerogel. This method consists of 3 steps; gel preparation, aging and drying. It is prepared using the solution of silica source and gelation occurs when the catalyst is added to solution. The resulting gels are called hydrogels, aquagel, alcogel and aerogel. The aging is the addition of new monomers to the silica mesh and the increase in the cross-linking degree of siloxane. Stability and strength of aerogels increase during aging and gel bindings are strengthened. The purpose of the drying process is to purify the gel from liquid pores and to protect the skeleton structure [6,7].
In this work, silica aerogel was synthesized by using natural silica source as perlite and quartz powders. Surface modification with TEOS was carried out in the aging phase to strength the bonds in the silica aerogel structure and to increase the strength of the material. The liquid of gel structure was removed by drying in atmospheric pressure during in the specified time at different temperatures and finally silica aerogel was produced at room temperature. Then, the microstructure of produced powders were analyzed by scanning electron microscopy (SEM) and Field Emission Scanning Electron Microscopy (FESEM) XRD (X-Ray Diffractometer) device was used to determine the phases in the produced powder material.
Perlite which is used as a silica source is a name given to naturally occurring silica-based volcanic rocks. The most important feature that distinguishes perlite from other volcanic dusts is that its original volume can be increased to about 20 times when it is heated around the softening temperature. When perlite is heated rapidly over 870 degrees, numerous pores are formed on the thermally expanding perlite. The porous structure of the thermally expanded perlite sample and its very light make it an excellent heat and sound insulation material [8].
2. Experimental
2.1. Materials
Perlite and quartz powders were used as silica source. The chemical composition of perlite and quartz powders is shown in Table 1. While the silica content in the perlite material is in the range of 71-75%, this ratio is up to 99% in the quartz powder. Sodium hydroxide (NaOH), n-heptane (C7H16), ethanol (C2H5OH), hydrochloric acid (HCl, 37%) were obtained from Merck Chemicals. Tetraethylorthosilicate (TEOS, 98%, (C₂H₅O)₄Si) chemical was supplied by Alfa Aesar. Distilled water was used in the steps of washing the gelatin and taking the solution during production.
2.2. Preparation of sodyum silicate solution
The flow chart of silica aerogel preparation from perlite and quartz powders are illustrated in Fig. 1. If we get reference to the perlite powder which we used as a raw material; 30 grams of perlite powder was mixed with 300 ml of 1 M NaOH solution in 500 ml Erlenmeyer. The prepared mixture was boiled in a heated stirrer for 1.5 hours at 100°C to dissolve the perlite powder in a large amount in the NaOH solution. The refluxed mixture was allowed to cool at room temperature for a while and then the mixture was filtered to remove undissolved residues. During the filtration process, 125 mm diameter filter papers from Chmlab Group were used. The sodium silicate solution was used for silica aerogel production. All of these processes were applied in the quartz powders. As a result of two different raw materials are used for producing silica based aerogels.
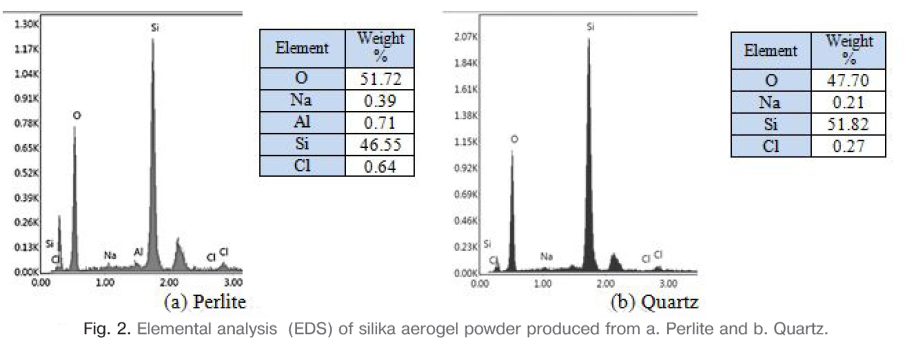
2.3. Production of silica aerogels
The prepared sodium silicate solution was used to prepare silica aerogel after resting. The pH of the solution was adjusted to 6-7 by neutralizing with 1M HCl acid solution prepared. The prepared gel was aged at the room temperature for 3 days. Then the aged gel was filtered and separated from the solution. The sodium nitrate residues in the gel were removed by washing distilled with water and thus the Na ratio was reduced to the minimum. The extent of Na removal was measured by analyzing Na-content of dried gel using EDS. The sodium content of the dry gel was about 0.39% for perlite and 0.21% for quartz are shown in Fig. 2. Aging process was continued by silica jel was soaked in a solution of 20 vol% Ethanol/H2O at 50°C for 1 day. A volume of 70% Ethanol/TEOS solution was prepared and the gel was soaked in this solution for 1 day at 60°C. The gel was washed several times with n-heptane to remove the ethanol/TEOS solution. Aerogels were aged in a solution of 70 vol% Ethanol/TEOS for 1 day at 50°C and room temperature. The modified gels were aged for another 24 h inside n-heptane at room temperature before air drying. Finally, the silica aerogel was dried in 1 day at 50°, 75° and 125°C with partially covered condition. The resulting silica aerogel powders were rested at room temperature for 1 day and then used for characterization.
2.4. Characterization
Microstructure and morphology of the silica aerogel powder was observed by field emission scanning electron misroscopy (FE-SEM). Elemental analysis was performed on aerogel powders using EDS (Energy Dispersive Spectroscopy) analysis. The phases of silica aerogel powders and the concentration, amount and crystal size of these phases were analyzed using XRD (X-ray Diffraction) technique. The weight loss of the aerogel powders were studied thermo gravimetric and differential thermal analysis (Netzsch STA 449) in N2 atmosphere at a heating rate of 5 °C.min-1 from room temperature to 1000°C. Fourier transform infrared spectroscopy (FTIR, Spectrum RX-1, Perkin Elmer) was employed to investigate the chemical bonding state of surface modifying agent with aerogels. The specific surface area and pore size distribution of aerogel were determined by Brunauer-Emmett-Teller (BET) method using Autosorb-1, Quantachrome surface area analyzer.
3. Results and discussion
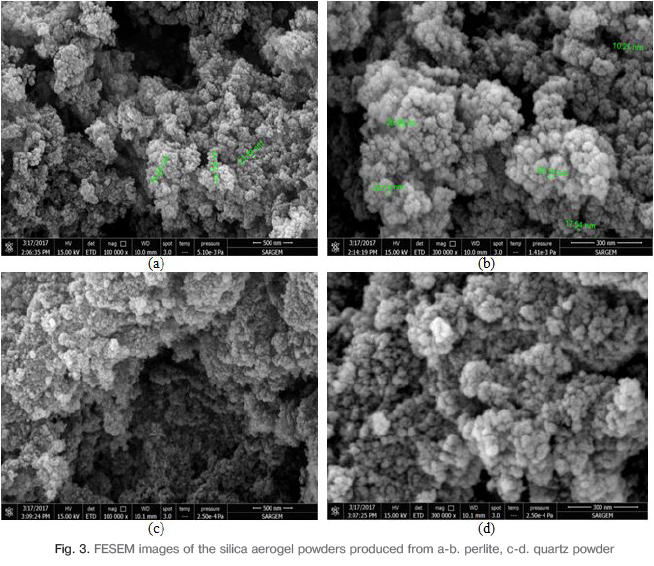
Since the particle structure of the obtained silica aerogel powders is in the nanoscale, the microstructures are not detailed by SEM and the microstructures are investigated with higher magnification FESEM device. The FESEM image of the microstructure at 100.000X and 300.000X magnification of the silica aerogel powder obtained from the perlite sample is shown in Fig.3a-b. and quartz sample is shown in Fig.3c-d. In the FESEM microstructure images, it was determined that the size of the powder grains is nano-sized. It has been found that the powder sizes are generally between 10 and 50 nm. Powder microparticles have sponge-like internal structure and a three dimensional cross-linked network. The average grain size of silica airgel was determined also by BET analysis.
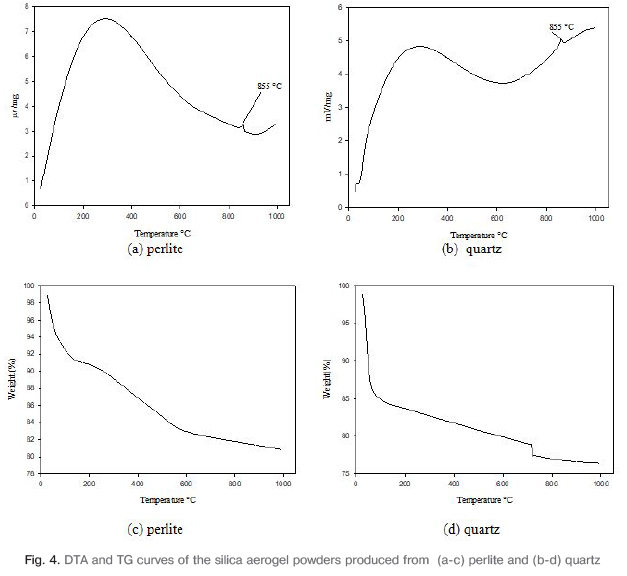
The thermal stability of the aerogel powders were investigated with thermo-gravimetric (TG) and differential thermal analyses (DTA). Fig.4a-b. shows the DTA curve of silica aerogel powders. Exothermic peak was observed at 855°C in the DTA curve because of oxidation. Exothermic peak formed with the oxidation of the methyl (-CH3) group in the structure.
Fig.4c-d. show the TG curves of silica aerogel powders. In the TG analysis graph of produced from perlite, weight loss is observed at a temperature range of 50-100°C because of water molecules adsorbed to the surface. The loss of weight in the large scale occurs due to the thermal decomposition of methyl groups in the range of 250-850°C. In the Fig. 4(d) graph, weight loss was observed in aerogel by evaporation of water molecules at the temperature range of 40-100°C. It is seen from graphs that there is more weight loss than aerogel from perlite powder. In the range of 600-800°C, loss of weight occurred due to dehydration and polycondensation of aerogels.
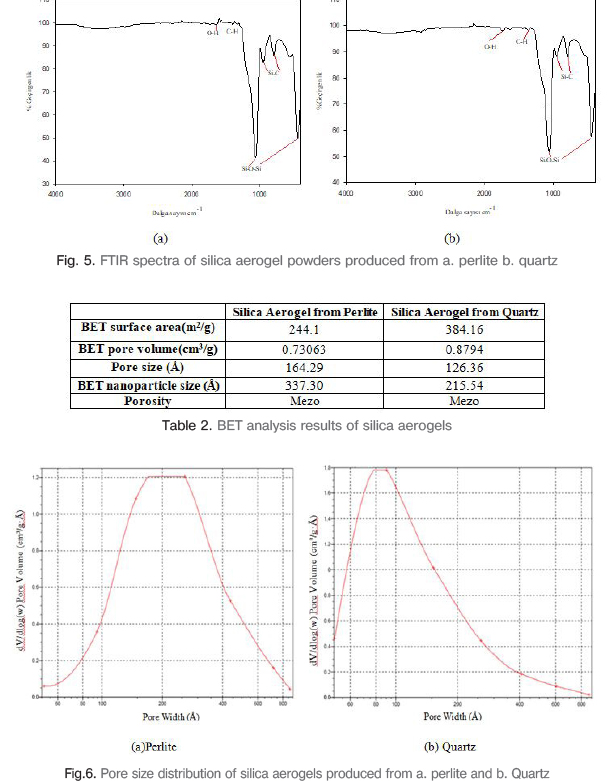
Figure 5 shows FTIR spectra of the aerogel powders. In fig.5(a), the ~443 and ~1061 cm-1 bands are associated with the Si-O-Si asymetric and symmetric bond streching vibration. These bonds are observed due to bond bending and streching vibration. Si-C peaks originated from vibrations at ~795 and ~955 cm-1. While C-H peaks were formed due to adsorption at ~1322 and ~1395 cm-1, O-H adsorption bonds occurred because of physically adsorbed water at ~1641 cm-1. In fig.5(b), Si-C bond peaks are at ~799 and ~960 cm-1, Si-O-Si strong and evident bonds are at ~454 and ~1070 cm-1, C-H bonds are at ~1375 and ~1455 cm-1 and finally -OH bond is occured at 1740 cm-1.
BET surface area, BET pore volume, pore size and BET nanoparticle size values of aerogels obtained from natural silica sources are given in Table 2. Figure 6 shows pore size distribution of silica aerogels. It has been understood that the pore size in the silica aerogel obtained from the perlite powder is in the range of 60-400 Å (6-40 nm) and is produced in the desired nanometer size. For silica aerogel obtained from quartz powder, pore diameter decreased to 5 nm in the graph of pore size distribution and nano-size aerogel powder production is provided.
4. Conclusion
The silica aerogel powder was prepared by the sol-gel method using the ambient pressure drying. According to the characterization results obtained in the research; it was found that silica aerogels have very low density due to the three-dimensional and porous network and high porosity values with porous spongy structures made up of millions of holes with FESEM analysis; endothermic peaks were observed from dehydration, decomposition reactions and exothermic peaks were observed from oxidation with DTA analysis. The TG curves of silica aerogel powders show that; the loss of weight in the large scale occurs due to the thermal decomposition of methyl groups. The FTIR analysis showed that Si-C peaks originated from vibration, strong and prominent Si-O-Si peaks originating from asymmetric and symmetrical bonds of molecules and movement of the bonds, C-H peaks originated from adsorption and O-H adsorption peaks originating from physically adsorbed water. Perlite and quartz based silica aerogels show high BET surface areas as 244 m2/g and 384 m2/g and average pore sizes as 16 nm and 12 nm. The present process is very suitable for effective silica aerogel powders production. The aerogel production from natural silica raw materials by ambient pressure drying method is very significant and useful for lots of industrial areas.
References
[1] S. Nagapriya, M.R. Ajith, H. Sreemoolanadhan, M. Mathew, S.C. Sharma, “Hydrophobic silica aerogels by ambient pressure drying”, Materials Science Forum, pp:476-479, 2015.
[2] Ming Li, Hongyi Jiang, Dong Xu, Ou Hai, Wei Zeng, “Low density and hydrophobic silica aerogels dried under ambient pressure using a new co-precursor method”, Journal of Non-Crystalline Solids, pp:187-193, 2016.
[3] G. Carlson, D. Lewis, K. McKinley, J. Richardson, T. Tillotson, “Aerogel commercialization: technology, markets and costs”, Journal of Non-Crystalline Solids, pp:372-379, 2000.
[4] N.Nazriati, Heru Setyawan, Samsudin Affandi, Minta Yuwana, Sugeng Winardi, “Using bagasse ash as a silica source when preparing silica aerogels via ambient pressure drying”, Journal of Non-Crystalline Solids, pp: 6-11, 2014.
[5] Poonam M. Shewale, A. Venkateswara Rao, A. Parvathy Rao, “Effect of different trimethyl silylating agents on the hyrophobic and physical properties of silica aerogels”, Applied Surface Science, pp:6902-6907, 2008.
[6] Duygu GÜLER, “Silis Kumu, Feldspat ve Tetraetilortosilikattan Soljel Yöntemi ile Silika Aerojel Sentezi ve Karakterizasyonu”, Yüksek Lisans Tezi, Gazi Üniversitesi Fen Bilimleri Fakültesi, 2009.
[7] A. Soleimani Dorcheh, M.H. Abbasi, “Silica aerogel; synthesis, properties and characterization”, Journal of Materials Processing Technology, pp: 10-26, 2008.
[8] https://tr.wikipedia.org/wiki/Perlit



